- E-mail : info_marketing@jindunchemical.cn
- Phone : +86 21 64057580
- Address : Shanghai China
Improved synthesis process for the diabetes drug troglitazone succinate: minimizing impurity generation
Troglitazone succinate, a new oral DPP-4 inhibitor developed by Takeda
Pharmaceuticals, has been approved for the treatment of type II diabetes in
Japan on March 26, 2015.
The activity data showed that troglitazone succinate inhibited DPP-4 more
effectively than Alogliptin, another type II diabetes drug developed by Takeda
Pharmaceuticals, and Sitagliptin (trade name: Genovel), a heavyweight
hypoglycemic drug owned by Merck Sharp & Dohme, and, moreover, the
selectivity of the drug was excellent.
It is because of the unique therapeutic effect and good market prospect of troglitazone succinate that pharmaceutical researchers have been improving its
preparation process and developing new preparation processes.
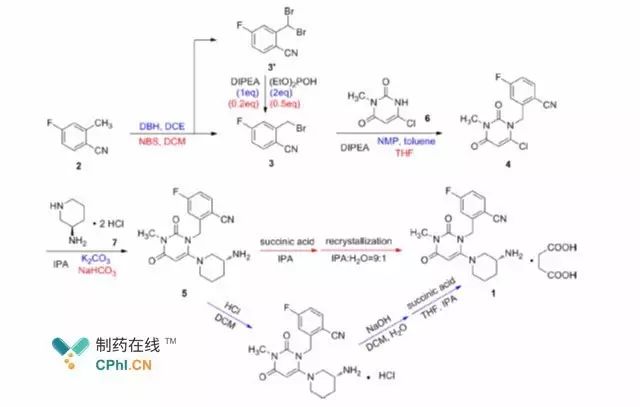
Figure 1 Synthesis process of troglitazone succinate
Takeda Pharmaceutical's synthetic process for troglitazone succinate is
shown in Figure 1. The route is based on 4-fluoro-2-methylbenzonitrile as the
starting material, and compound 3 is obtained after bromination; compound 3 can
be condensed with intermediate 6 to obtain compound 4, and further condensed
with compound 7 to obtain troglitazone, which can be successfully prepared by
further acidification and recrystallization of troglitazone succinate.
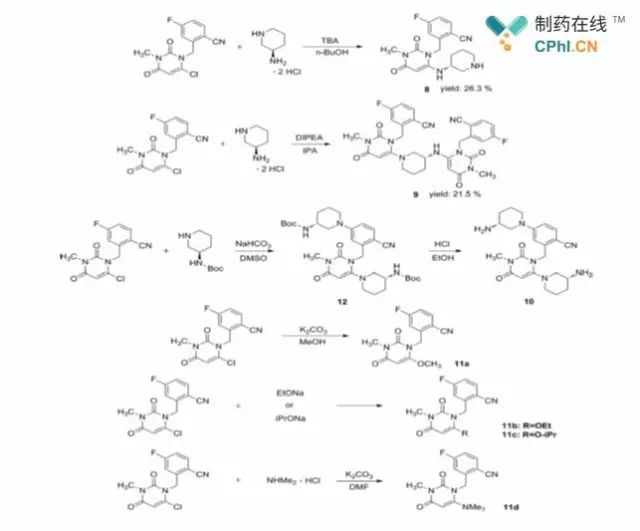
Figure 2 Synthesis of compounds 8-11
Researchers noted that the nucleophilic substitution step is key to obtain
high regioselectivity for the reaction. For the condensation of compound 4 with
compound 7, in order to avoid the formation of the regional isomer 8 (Figure 2),
some researchers had reported in 2016 an optimization improvement study of this
step using N-protected compound 7 condensed with compound 4, but it was
difficult to avoid the formation of by-products such as cyanohydrolysis products
during deprotection. In the new study, the researchers optimized the conditions
of several steps, including this step.
In previous reports, the initial raw material 2 was reacted with 1
equivalent of 1,3-dibromo-5,5-dimethylethanoylurea in DCE solvent to give mainly
product 3', which was then stripped of one molecule of HBr under diethyl
phosphite, DIPEA conditions to give compound 3. In the improved process, the
researchers successfully replaced a class of toxic solvents, DCE, with a benign
solvent, DCM, and by adjusting the equivalents, etc., to achieve the one-pot
preparation of compound 3. Subsequently, the researchers studied the
nucleophilic substitution more systematically, and the impurities formed in this
step are mainly compound 8, compound 9 and compound 10 shown in Figure 2; when
the protonic solvent (MeOH, EtOH, i-PrOH or DMF) is used for this reaction,
impurities 11a-11d are formed. to better study these impurities, the researchers
also synthesized them specifically (Figure 2).
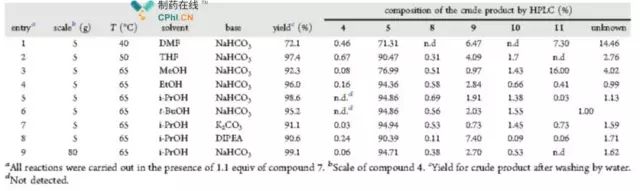
Figure 3 Nucleophilic substitution condition screening
As shown in Figure 3, the researchers systematically screened the nucleophilic substitution step for conditions such as reaction temperature, solvent and base use, and performed systematic impurity analysis using HPLC means. It was found that when sodium bicarbonate was used as the basic catalyst, isopropanol as the solvent, and the reaction temperature was 65°C, the reaction impurities could be reduced to a minimum with a yield of 99.1% (entry 9).
The purity of the crude product of troglitazone synthesized using the improved process was up to 94%, which was a great improvement over the original process. After further acidification and recrystallization, trigliptin succinate with a purity of 99.93% was successfully obtained.
In conclusion, the new process has successfully developed an efficient and precise route for the synthesis of the diabetes drug troglitazone succinate, reducing many impurities in the original process to very low limits and successfully avoiding the use of toxic solvents such as 1,2-dichloroethane. This will undoubtedly be of great benefit in meeting the expanding market for troglitazone succinate.
-
date
2022-10-10
-
location
Shanghai, China