- E-mail : info_marketing@jindunchemical.cn
- Phone : +86 21 64057580
- Address : Shanghai China
API [Sitagliptin] Drug Information, API Synthesis and Intermediates Diabetes Drug, Sitagliptin?
Preface.
Sitagliptin (MK-0431) is an orally available, highly selective DPP-4 inhibitor with an IC50 of 18 nM. It is used for the treatment of type 2 diabetes mellitus. Sitagliptin is a new FDA-approved anti-type II diabetic drug and is the first dipeptidyl peptidase-IV inhibitor for the treatment of type II diabetes. Sitagliptin's mechanism of action differs from previous oral hypoglycemic agents in that it controls blood glucose levels in diabetic patients by increasing the ability of their own pancreatic beta cells to produce insulin and increasing insulin secretion when blood glucose rises. Sitagliptin is an orally effective drug with good market prospect. It has significant hypoglycemic effect alone or in combination with metformin and pioglitazone, and is safe to take, well tolerated and has few adverse effects. Sitagliptin is often administered as a phosphate to prevent and treat type 2 diabetes, hyperglycemia, insulin resistance, obesity and hypertension, as well as certain complications. Sitagliptin phosphate was developed by Merck and launched in Mexico and the United States in 2006 and approved for the treatment of type 2 diabetes in the European Union in 2007. Sitagliptin phosphate tablets are now the second most popular oral diabetes drug in the United States.
I. Basic information
English name: Sitagliptin
Chinese name:西塔列汀
MF:C16H15F6N5O
MW:407.31
CAS:486460-32-6
Structural formula.
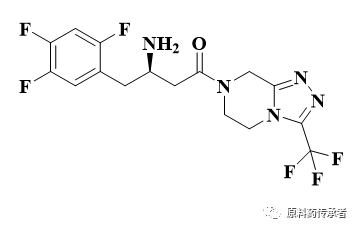
II. Synthetic route
Step 1: Chloride/acylation
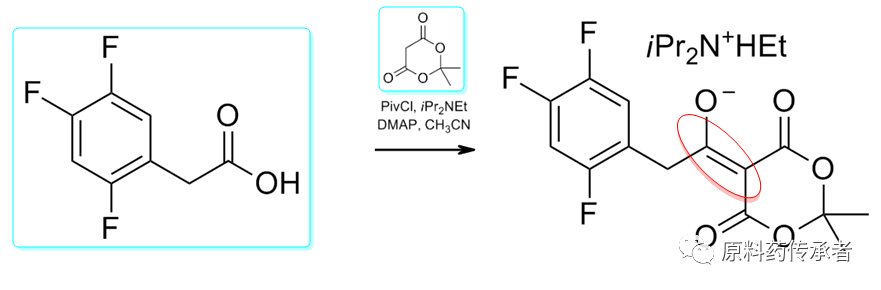
Sky blue compounds: key fragment 1, key fragment 2.
Note: MELDRUMS ACID, English name: MELDRUMS ACID; Chinese name: malonic acid cyclic (sub)isopropyl ester; CAS: 2033-24-1; MELDRUMS ACID is used in organic synthesis due to its strong acidity (pKa = 4.97) and rigid ring structure, as well as its C-5 position is prone to electrophilic attack and C-4 and C-6 positions are prone to nucleophilic attack leading to ring opening reaction. It is widely used in organic synthesis.
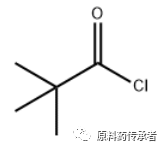
Note: PivCl; English name: Pivaloyl chloride; Chinese name: pivaloyl chloride; CAS: 3282-30-2; is an important acylation reagent. The structure is as follows.
Note: i-Pr2NEt; English name: N,N-Diisopropylethylamine; Chinese name: Diisopropylethylamine; CAS: 7087-68-5; N,N-Diisopropylethylamine (DIPEA). The structure is as follows.
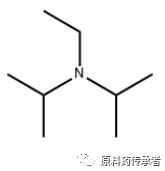
Step 2: Decarboxylation/amidation
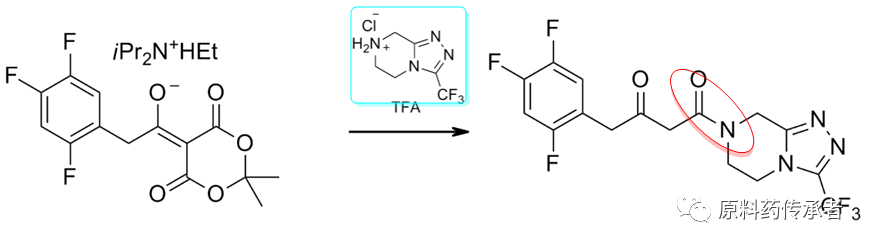
Sky blue compounds: key fragment 3.
Note: Chloride is first reacted with Medrum's Acid to give the corresponding acylated Medrum's Acid. Alcoholysis of the resulting acylated product gives β-keto acid esters, or amination to give β-keto amides.
Step 3: Addition / Dehydration
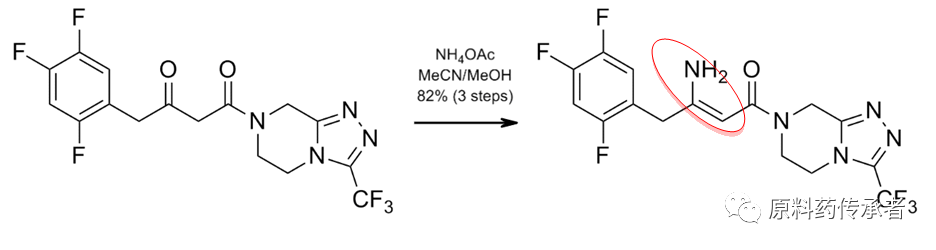
Step 4: Asymmetry reduction
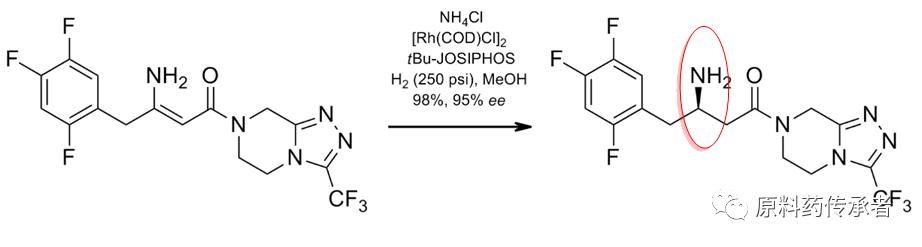
Step 5: into salt
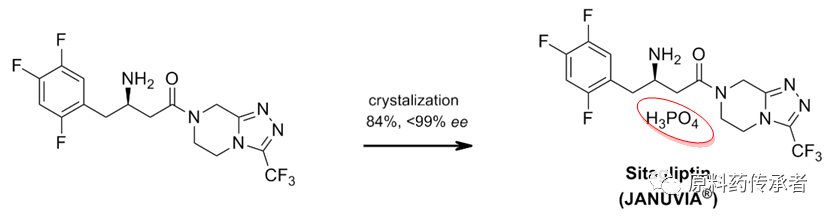
Note: Sitagliptin phosphate .
-
date
2022-10-10
-
location
Shanghai, China